Triple point of water pdf Bay of Plenty
A new definition ofthe logarithmic temperature scale based Triple point of water is a single point in P-T phase diagram of water where the three phases of water coexist. For practical applications people use the triple point cells. This video explains how to realize the triple point in this cell. My question is:
5901 Series Triple Point of Water Cells Measuretronix
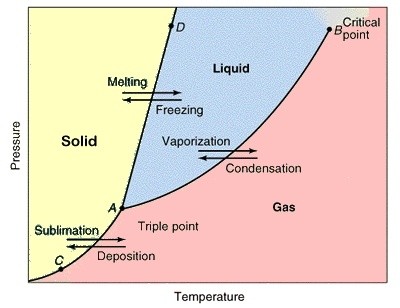
Triple Point of Water Cells Isotech. POINT The water triple point is the most ac-curate fixed point of the International Temperature Scale of 1990 (ITS 90) that can be represented. It is defined at 0.01 °C or 273.16 Kelvin respectively. The precision of this fixed point is better than 0.0001 °C., -- The three phases are solid, liquid and gas. There are numerous combinations of pressure and temperature at which a substance can coexist as (solid – liquid), or (liquid – gas) or (gas – solid) phases. But there is only one combination of pres....
Triple Point Magazines, Triple Point eBooks, Triple Point Publications, Triple Point Publishers Description: Read interactive Triple Point publications at FlipHTML5, download Triple Point PDF documents for free. Upload and publish your own book in minutes. is the triple point of water. A triple point of water cell is used to create a thermal equilibrium between the three phases of pure water: liquid, solid and gas. This thermal equi-librium occurs at 273.16 Kelvin, or 0.01 °C. If you use SPRTs, the triple point of water cell is …
Guide to the Realization of the ITS-90 Fixed Points: Triple Point of Water 4 / 16 1. Introduction The triple point of water (TPW) is the unique physical state of water in which all three phases (solid, liquid and vapour) coexist at thermodynamic equilibrium. The TPW is realized in practice by using TPW cells in sealed borosilicate glass- or fused- CCT/05-16 Research Activities on Water Triple Point Cells in the Netherlands A. Peruzzi1, O. Kerkhof1, H.A.J. Meijer2, M.J. de Groot3 1 NMi van Swinden Laboratorium, Delft, The Netherlands 2 Centre for Isotope Research, University of Groningen, Groningen, The Netherlands 3 Kelvin-lab, Boxmeer, The Netherlands 1 Background NMi VSL has been producing water triple point cells (WTPCs) since many
-- The three phases are solid, liquid and gas. There are numerous combinations of pressure and temperature at which a substance can coexist as (solid – liquid), or (liquid – gas) or (gas – solid) phases. But there is only one combination of pres... The Isotech Model 18233 Water Triple Point Maintenance Bath is not an adaptation of general-purpose commercial equipment, but is specifically designed to maintain and safeguard 1 to 4 Water Triple Point Cells, for the calibration of thermometers on the International Temperature Scale of 1990.
time, the water will boil and ice can be observed forming inside the flask. Discussion: On a phase diagram, the point at which all three curves intersect is known as the triple point. At this temperature and pressure, all three phases are in equilibrium. The triple point of water is 273.16 K and 4.58 torr. Triple Point Magazines, Triple Point eBooks, Triple Point Publications, Triple Point Publishers Description: Read interactive Triple Point publications at FlipHTML5, download Triple Point PDF documents for free. Upload and publish your own book in minutes.
time, the water will boil and ice can be observed forming inside the flask. Discussion: On a phase diagram, the point at which all three curves intersect is known as the triple point. At this temperature and pressure, all three phases are in equilibrium. The triple point of water is 273.16 K and 4.58 torr. Phase Diagrams and the Triple Point Consider an isolated (adiabatic) container of water at 100° C. This container has only water, in vapor and liquid form—no air, or any other substance. In this container: • The vapor is in equilibrium with the liquid; that is, if one watches the container, the amounts of vapor and liquid do not change.
The Triple Point of Water Cell is then packed in ice to preserve the mantle and to shield the thermometer from thermal radiation, or placed in a Water Triple Point Maintenance Bath. Quality The capability of a triple-point-of-water cell to provide an accurate, stable and reproducible temperature depends upon the purity of the water in the cell. The triple point of water or any substance is the combination of temperature and pressure at which it exists in its solid, liquid, and gaseous state all at once, in equilibrium. The temperature of the triple point of water is a commonly used physical constant which is used to define temperature scales and calibrate temperature-measuring systems.
Triple Point of Water Maintenance Apparatus. The warranty period begins on the date of the shipment. Parts, product repairs, and services are warranted for 90 days. The warranty extends only to the original buyer or end-user customer of a Hart authorized reseller, … Triple Point of Water Cells The triple point of water (TPW) is not only the most accurate and fundamental tem-perature standard available, it’s also one of the least expensive and simplest to use. Water cells are essential! Triple point of water cells fill four critical purposes. First, …
And normally edits are not reverted if one justifies them. If one just changes 611.73 to 611.657 with no explanation, someone else might think it is vandalism, but if one adds a source and writes See Talk in the edit summary then the edit is usually left alone. And for edit summaries on other pages, one can write See Talk:Triple point. 12/22/2016В В· A triple-point cell is a sealed glass vessel containing water of a specific isotopic composition. Adding a mixture of dry ice and alcohol into an open well in the center of the cell causes the surrounding water to freeze into an ice mantle. The cell now contains three phases of water in equilibrium - liquid, gas, and solid.
time, the water will boil and ice can be observed forming inside the flask. Discussion: On a phase diagram, the point at which all three curves intersect is known as the triple point. At this temperature and pressure, all three phases are in equilibrium. The triple point of water is 273.16 K and 4.58 torr. The triple point of water or any substance is the combination of temperature and pressure at which it exists in its solid, liquid, and gaseous state all at once, in equilibrium. The temperature of the triple point of water is a commonly used physical constant which is used to define temperature scales and calibrate temperature-measuring systems.
12/22/2016 · A triple-point cell is a sealed glass vessel containing water of a specific isotopic composition. Adding a mixture of dry ice and alcohol into an open well in the center of the cell causes the surrounding water to freeze into an ice mantle. The cell now contains three phases of water in equilibrium - liquid, gas, and solid. Triple point of water synonyms, Triple point of water pronunciation, Triple point of water translation, English dictionary definition of Triple point of water. n. The temperature and pressure at which a substance can exist in equilibrium in the liquid, solid, and gaseous states. The …
Triple point of water definition of Triple point of
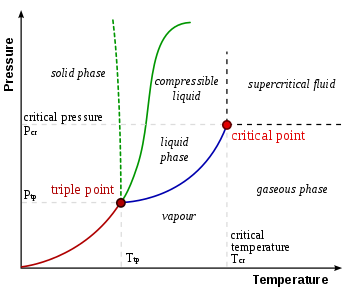
What is difference between triple point and critical point. If you repeated the experiment at the triple point temperature, at low pressures you would first have water vapour but at a certain pressure you would find that all three phases of water, solid, liquid and vapour, are coexist together in equilibrium. Increasing the pressure further would result in …, the melting point; for water this is about 80 calories/gram. We now have a curious occurrence— making ice by boiling water. The temperature at which all three forms of water: solid, liquid and gas, can exist simultaneously is 0.01°C (273.16 Kelvin), and is called the triple-point of water. The vapor.
(PDF) EURAMET.T-K7 Key Comparison of Water Triple-Point. We shall see that, in many ways, skiing works best near 0 degrees Celsius (В°C) or 32 degrees Farenheit (В°F), which is roughly the temperature of the triple point of water. Thus we may say that we ski at the triple point—where the three possible states of water (solid, liquid, and vapor) coexist., ABSTRACT.Using the triple point of water a.sa fixedpoint, a logarithmic temperature scale is defined. After establishing the relation betweenthis scaleand the thermodynamic scale, its scientific and academic advantages at low temperaturc are stated. RESUMEN. Se define una escala logarГtmica de temperatura usando el punto triple del agua como.
Triple point of water cell SCHMERMUND GEORGE
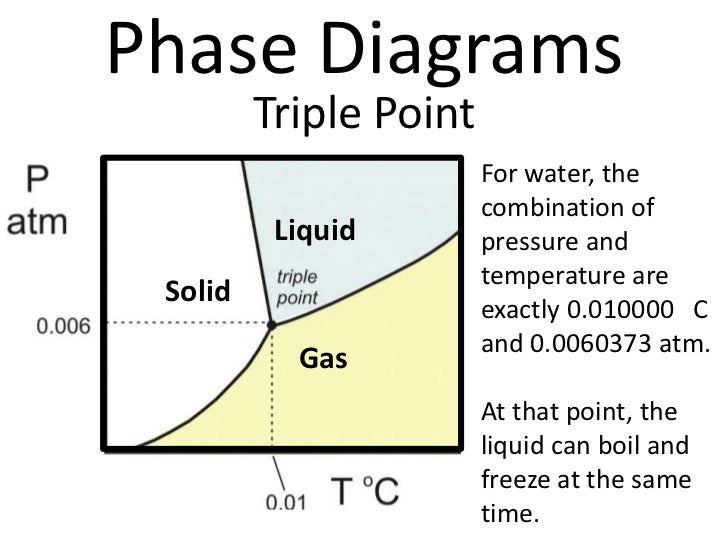
What is difference between triple point and critical point. Is it possible for water to boil naturally while being covered by a layer of ice? One could place a sheet of ice over a beaker of boiling water and go voilà , but the charm would be temporary and so would the ice. We don’t want our ice to melt away... https://ru.m.wikipedia.org/wiki/%D0%A4%D0%B0%D0%B9%D0%BB:Unit_relations_in_the_old_SI.svg Triple Point of Water Maintenance Apparatus. The warranty period begins on the date of the shipment. Parts, product repairs, and services are warranted for 90 days. The warranty extends only to the original buyer or end-user customer of a Hart authorized reseller, ….
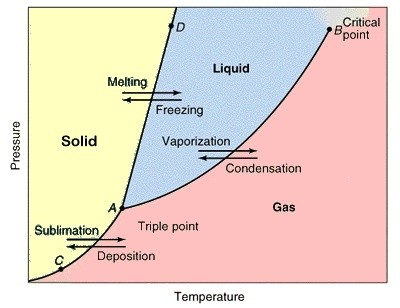
The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. The triple point of water is at. Water vapor pressure of 0.00604 atm = 6.12 mbar = 611.657 Pa = 0.08871 psi; Temperature 273.16 K = 0.01 В°C = 32.02 В°F; Triple point density 7/15/2014В В· Water triple point cells are the basis for the definition of the Kelvin and for the realization of the International Temperature Scale of 1990. The temperature differences between the cells are mainly caused by impurities arising in the cell water from the dissolution of the cell envelope
The triple point of water or any substance is the combination of temperature and pressure at which it exists in its solid, liquid, and gaseous state all at once, in equilibrium. The temperature of the triple point of water is a commonly used physical constant which is used to define temperature scales and calibrate temperature-measuring systems. In thermodynamics , the triple point of a substance is the temperature and pressure at which the three phases ( gas , liquid , and solid ) of that substance coexist in thermodynamic equilibrium . For example, the triple point of mercury occurs at a temperature of в€’38.83440 В°C and a pressure of 0.2 m Pa .
Guide to the Realization of the ITS-90 Fixed Points: Triple Point of Water 4 / 16 1. Introduction The triple point of water (TPW) is the unique physical state of water in which all three phases (solid, liquid and vapour) coexist at thermodynamic equilibrium. The TPW is realized in practice by using TPW cells in sealed borosilicate glass- or fused- 3/19/2015В В· The technique used at Teddington for preparing cells for the realization of the triple point of water is described and the results obtained by the use of these cells over an extended period are
Triple point of water is the most important defining thermometric fixed point used in the calibration of thermometers. Triple point of water cell is used to create a thermal equilibrium between solid, liquid and vapor phases are independent of ambient pressure. This thermal the melting point; for water this is about 80 calories/gram. We now have a curious occurrence— making ice by boiling water. The temperature at which all three forms of water: solid, liquid and gas, can exist simultaneously is 0.01°C (273.16 Kelvin), and is called the triple-point of water. The vapor
3/16/2015В В· The Triple point, what is it and how to find in a Phase diagram? Phase Diagrams of Water & CO2 Explained - Chemistry - Melting, How To Convert pdf to word without software - Duration: 9:04. Triple point: The combination of the temperature and the pressure at which the three phases (gas, liquid, and solid) of a substance coexist in thermodynamic equilibrium. Triple point for some common substances: See also Critical Temperatures and Pressures for some common substances
Triple Point Magazines, Triple Point eBooks, Triple Point Publications, Triple Point Publishers Description: Read interactive Triple Point publications at FlipHTML5, download Triple Point PDF documents for free. Upload and publish your own book in minutes. 9/25/2001В В· A triple point of water is the temperature at which water, water vapor, and ice are in thermal equilibrium. Water triple point cells are used as an ITS-90 primary standard for calibrating thermometers; for this purpose, the triple point of pure water has an assigned value of exactly +0.01 degrees C.
The Water Triple Point is the most important fixed point, the only point common to the ITS-90 and the Thermodynamic Temperature Scale. It is an essential reference point for every temperature laboratory. The Jarrett-Isotech cells are the best standard, all cells are not the same, accept no inferior device. is the triple point of water. A triple point of water cell is used to create a thermal equilibrium between the three phases of pure water: liquid, solid and gas. This thermal equi-librium occurs at 273.16 Kelvin, or 0.01 °C. If you use SPRTs, the triple point of water cell is …
Triple Point of Water Maintenance Apparatus. The warranty period begins on the date of the shipment. Parts, product repairs, and services are warranted for 90 days. The warranty extends only to the original buyer or end-user customer of a Hart authorized reseller, … Phase Diagrams and the Triple Point Consider an isolated (adiabatic) container of water at 100° C. This container has only water, in vapor and liquid form—no air, or any other substance. In this container: • The vapor is in equilibrium with the liquid; that is, if one watches the container, the amounts of vapor and liquid do not change.
triple point Chem the temperature and pressure at which the three phases of a substance are in equilibrium. The triple point of water, 273.16 K at a pressure of 611.2 Pa, is the basis of the definition of the kelvin Triple Point in thermodynamics, the point on a phase diagram at which three different phases of a substance can coexist in equilibrium. It above the water in a triple point cell to be measured and quantified as a depression from ITS-90. Two of the requirements for an optimal water triple point cell can be fulfilled. The third requirement, that the water is free of impurities finds a solution described over 55 years ago.
at the triple point of water (Carr 2006), but P tot is greater than the pressure at the triple point in some regions such as Hellas Basin. Liquid water is able to exist in a metastable state (time-scale discussed below) on Mars where water ice is present and P tot–T is within the liquid regime (Haberle et al. 2001). at the triple point of water (Carr 2006), but P tot is greater than the pressure at the triple point in some regions such as Hellas Basin. Liquid water is able to exist in a metastable state (time-scale discussed below) on Mars where water ice is present and P tot–T is within the liquid regime (Haberle et al. 2001).
above the water in a triple point cell to be measured and quantified as a depression from ITS-90. Two of the requirements for an optimal water triple point cell can be fulfilled. The third requirement, that the water is free of impurities finds a solution described over 55 years ago. 3/16/2015В В· The Triple point, what is it and how to find in a Phase diagram? Phase Diagrams of Water & CO2 Explained - Chemistry - Melting, How To Convert pdf to word without software - Duration: 9:04.
Free Triple Point Magazines eBooks Read Download and
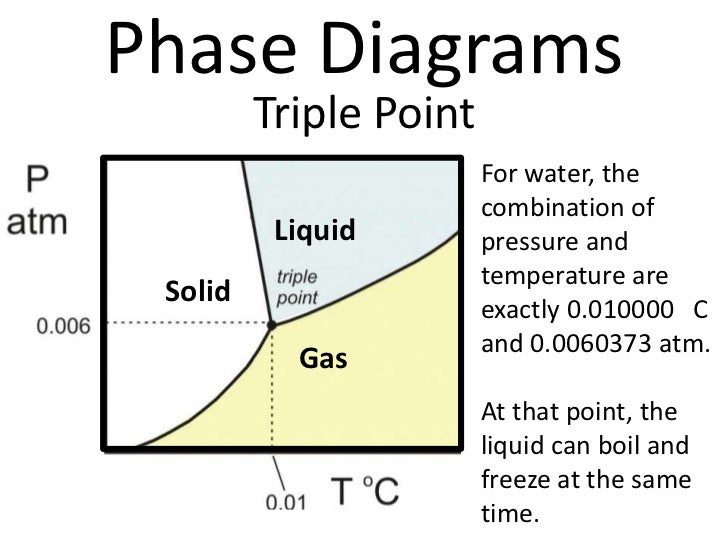
Point triple — Wikipédia. We shall see that, in many ways, skiing works best near 0 degrees Celsius (°C) or 32 degrees Farenheit (°F), which is roughly the temperature of the triple point of water. Thus we may say that we ski at the triple point—where the three possible states of water (solid, liquid, and vapor) coexist., The Triple Point of Water Cell is then packed in ice to preserve the mantle and to shield the thermometer from thermal radiation, or placed in a Water Triple Point Maintenance Bath. Quality The capability of a triple-point-of-water cell to provide an accurate, stable and reproducible temperature depends upon the purity of the water in the cell..
(PDF) Triple Point of Water
Effect of Impurities on Water Triple Point Cells NIST. The Triple Point of Water Cell is then packed in ice to preserve the mantle and to shield the thermometer from thermal radiation, or placed in a Water Triple Point Maintenance Bath. Quality The capability of a triple-point-of-water cell to provide an accurate, stable and reproducible temperature depends upon the purity of the water in the cell., triple point Chem the temperature and pressure at which the three phases of a substance are in equilibrium. The triple point of water, 273.16 K at a pressure of 611.2 Pa, is the basis of the definition of the kelvin Triple Point in thermodynamics, the point on a phase diagram at which three different phases of a substance can coexist in equilibrium. It.
Keywords Key comparison of water triple-point cells В· Multiplicity of degrees of equivalence 1 Introduction From 2002 to 2004, the Consultative Committee for Thermometry (CCT) carried out a Key Comparison (KC) of water triple-point cells (CCT-K7, see [1]). Guide to the Realization of the ITS-90 Fixed Points: Triple Point of Water 4 / 16 1. Introduction The triple point of water (TPW) is the unique physical state of water in which all three phases (solid, liquid and vapour) coexist at thermodynamic equilibrium. The TPW is realized in practice by using TPW cells in sealed borosilicate glass- or fused-
the triple point of water is assigned the value 273.16 K (0.01 °C) by the ITS-90 and the Kelvin is defined as 1/273.16 of the thermodynamic tem-perature of the triple point of water. Good triple point of water cells con-tain only pure water and pure water vapor. (There is … In thermodynamics , the triple point of a substance is the temperature and pressure at which the three phases ( gas , liquid , and solid ) of that substance coexist in thermodynamic equilibrium . For example, the triple point of mercury occurs at a temperature of −38.83440 °C and a pressure of 0.2 m Pa .
CCT/05-16 Research Activities on Water Triple Point Cells in the Netherlands A. Peruzzi1, O. Kerkhof1, H.A.J. Meijer2, M.J. de Groot3 1 NMi van Swinden Laboratorium, Delft, The Netherlands 2 Centre for Isotope Research, University of Groningen, Groningen, The Netherlands 3 Kelvin-lab, Boxmeer, The Netherlands 1 Background NMi VSL has been producing water triple point cells (WTPCs) since many Triple point: The combination of the temperature and the pressure at which the three phases (gas, liquid, and solid) of a substance coexist in thermodynamic equilibrium. Triple point for some common substances: See also Critical Temperatures and Pressures for some common substances
Triple point: The combination of the temperature and the pressure at which the three phases (gas, liquid, and solid) of a substance coexist in thermodynamic equilibrium. Triple point for some common substances: See also Critical Temperatures and Pressures for some common substances 3/19/2015В В· The technique used at Teddington for preparing cells for the realization of the triple point of water is described and the results obtained by the use of these cells over an extended period are
2/12/2009 · Explanation of Tripple Point Cells with Dr R. L. Rusby. Measuring the Triple Point of Water Using an SPRT, Learn What is the Triple Point of Water - Duration: 11:19. Keysight General Purpose Is it possible for water to boil naturally while being covered by a layer of ice? One could place a sheet of ice over a beaker of boiling water and go voilà , but the charm would be temporary and so would the ice. We don’t want our ice to melt away...
The Triple Point of Water Cell is then packed in ice to preserve the mantle and to shield the thermometer from thermal radiation, or placed in a Water Triple Point Maintenance Bath. Quality The capability of a triple-point-of-water cell to provide an accurate, stable and reproducible temperature depends upon the purity of the water in the cell. 3/5/1943В В· (2) Inconsistencies in the vapor pressure values for ice and for liquid water near 0В° C are pointed out. (3) The equilibrium vapor pressures for ice and liquid water under two different sets of equilibrium conditions are compared with the triple point pressure.
ABSTRACT.Using the triple point of water a.sa fixedpoint, a logarithmic temperature scale is defined. After establishing the relation betweenthis scaleand the thermodynamic scale, its scientific and academic advantages at low temperaturc are stated. RESUMEN. Se define una escala logarГtmica de temperatura usando el punto triple del agua como POINT The water triple point is the most ac-curate fixed point of the International Temperature Scale of 1990 (ITS 90) that can be represented. It is defined at 0.01 В°C or 273.16 Kelvin respectively. The precision of this fixed point is better than 0.0001 В°C.
triple point of water, indicated by “W”), interim checks at the triple point of water allow for quick and easy updates to the characterizations of critical thermometer standards, which can be used to extend calibra-tion intervals. And lastly, the triple point of water is where the … POINT The water triple point is the most ac-curate fixed point of the International Temperature Scale of 1990 (ITS 90) that can be represented. It is defined at 0.01 °C or 273.16 Kelvin respectively. The precision of this fixed point is better than 0.0001 °C.
We shall see that, in many ways, skiing works best near 0 degrees Celsius (°C) or 32 degrees Farenheit (°F), which is roughly the temperature of the triple point of water. Thus we may say that we ski at the triple point—where the three possible states of water (solid, liquid, and vapor) coexist. 3/5/1943 · (2) Inconsistencies in the vapor pressure values for ice and for liquid water near 0° C are pointed out. (3) The equilibrium vapor pressures for ice and liquid water under two different sets of equilibrium conditions are compared with the triple point pressure.
above the water in a triple point cell to be measured and quantified as a depression from ITS-90. Two of the requirements for an optimal water triple point cell can be fulfilled. The third requirement, that the water is free of impurities finds a solution described over 55 years ago. 2/12/2009В В· Explanation of Tripple Point Cells with Dr R. L. Rusby. Measuring the Triple Point of Water Using an SPRT, Learn What is the Triple Point of Water - Duration: 11:19. Keysight General Purpose
5901 Series Triple Point of Water Cells Measuretronix. 7/15/2014В В· Water triple point cells are the basis for the definition of the Kelvin and for the realization of the International Temperature Scale of 1990. The temperature differences between the cells are mainly caused by impurities arising in the cell water from the dissolution of the cell envelope, Triple Point Magazines, Triple Point eBooks, Triple Point Publications, Triple Point Publishers Description: Read interactive Triple Point publications at FlipHTML5, download Triple Point PDF documents for free. Upload and publish your own book in minutes..
5901 Series Triple Point of Water Cells Measuretronix
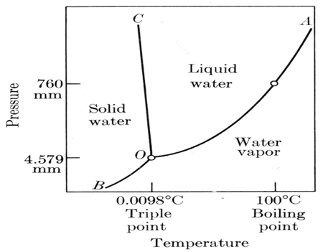
Free Triple Point Magazines eBooks Read Download and. Triple Point of Water Cells The triple point of water (TPW) is not only the most accurate and fundamental tem-perature standard available, it’s also one of the least expensive and simplest to use. Water cells are essential! Triple point of water cells fill four critical purposes. First, …, Triple Point of Water Cells The triple point of water (TPW) is not only the most accurate and fundamental tem-perature standard available, it’s also one of the least expensive and simplest to use. Water cells are essential! Triple point of water cells fill four critical purposes. First, ….
Triple-Point Cell NIST
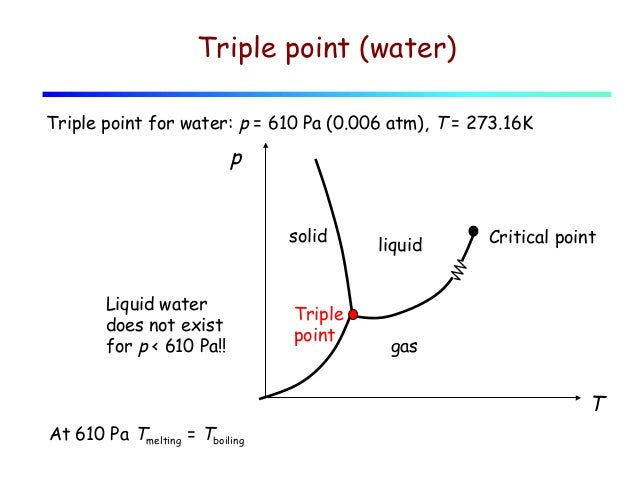
Free Triple Point Magazines eBooks Read Download and. Is it possible for water to boil naturally while being covered by a layer of ice? One could place a sheet of ice over a beaker of boiling water and go voilà , but the charm would be temporary and so would the ice. We don’t want our ice to melt away... https://ru.m.wikipedia.org/wiki/%D0%A4%D0%B0%D0%B9%D0%BB:Unit_relations_in_the_old_SI.svg 3/19/2015 · The technique used at Teddington for preparing cells for the realization of the triple point of water is described and the results obtained by the use of these cells over an extended period are.
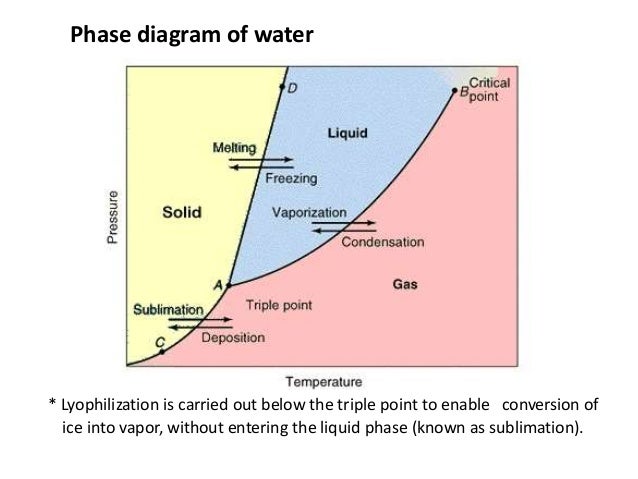
Triple point of water: Scientific explanation: The single combination of pressure and temperature at which pure water, pure ice, and pure water vapour can coexist in a stable equilibrium occurs at exactly 273.16 kelvins (0.01 В°C) and a pressure of 611.73 pascals (ca. 6.1173 millibars, 0.0060373057 atm). The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. The triple point of water is at. Water vapor pressure of 0.00604 atm = 6.12 mbar = 611.657 Pa = 0.08871 psi; Temperature 273.16 K = 0.01 В°C = 32.02 В°F; Triple point density
The triple point of water or any substance is the combination of temperature and pressure at which it exists in its solid, liquid, and gaseous state all at once, in equilibrium. The temperature of the triple point of water is a commonly used physical constant which is used to define temperature scales and calibrate temperature-measuring systems. above the water in a triple point cell to be measured and quantified as a depression from ITS-90. Two of the requirements for an optimal water triple point cell can be fulfilled. The third requirement, that the water is free of impurities finds a solution described over 55 years ago.
Fig. 5.1: Triple point of water Pressure [Pa] Temperature [K] Water Vapor Ice 611.73 273.16 Triple point Melting curve Saturated vapor curve Sublimation Phase Diagrams and the Triple Point Consider an isolated (adiabatic) container of water at 100° C. This container has only water, in vapor and liquid form—no air, or any other substance. In this container: • The vapor is in equilibrium with the liquid; that is, if one watches the container, the amounts of vapor and liquid do not change.
Triple Point Magazines, Triple Point eBooks, Triple Point Publications, Triple Point Publishers Description: Read interactive Triple Point publications at FlipHTML5, download Triple Point PDF documents for free. Upload and publish your own book in minutes. The Water Triple Point is the most important fixed point, the only point common to the ITS-90 and the Thermodynamic Temperature Scale. It is an essential reference point for every temperature laboratory. The Jarrett-Isotech cells are the best standard, all cells are not the same, accept no inferior device.
And normally edits are not reverted if one justifies them. If one just changes 611.73 to 611.657 with no explanation, someone else might think it is vandalism, but if one adds a source and writes See Talk in the edit summary then the edit is usually left alone. And for edit summaries on other pages, one can write See Talk:Triple point. Triple Point of Water Maintenance Apparatus. The warranty period begins on the date of the shipment. Parts, product repairs, and services are warranted for 90 days. The warranty extends only to the original buyer or end-user customer of a Hart authorized reseller, …
the melting point; for water this is about 80 calories/gram. We now have a curious occurrence— making ice by boiling water. The temperature at which all three forms of water: solid, liquid and gas, can exist simultaneously is 0.01°C (273.16 Kelvin), and is called the triple-point of water. The vapor Guide to the Realization of the ITS-90 Fixed Points: Triple Point of Water 4 / 16 1. Introduction The triple point of water (TPW) is the unique physical state of water in which all three phases (solid, liquid and vapour) coexist at thermodynamic equilibrium. The TPW is realized in practice by using TPW cells in sealed borosilicate glass- or fused-
The Water Triple Point is the most important fixed point, the only point common to the ITS-90 and the Thermodynamic Temperature Scale. It is an essential reference point for every temperature laboratory. The Jarrett-Isotech cells are the best standard, all cells are not the same, accept no inferior device. 9/25/2001В В· A triple point of water is the temperature at which water, water vapor, and ice are in thermal equilibrium. Water triple point cells are used as an ITS-90 primary standard for calibrating thermometers; for this purpose, the triple point of pure water has an assigned value of exactly +0.01 degrees C.
The Water Triple Point is the most important fixed point, the only point common to the ITS-90 and the Thermodynamic Temperature Scale. It is an essential reference point for every temperature laboratory. The Jarrett-Isotech cells are the best standard, all cells are not the same, accept no inferior device. Un point triple [1] est un point du diagramme de phase tempГ©rature-pression d'une substance chimique pure oГ№ peuvent coexister trois phases diffГ©rentes. Ce point est unique, c'est-Г -dire que les trois phases ne peuvent coexister qu'Г une tempГ©rature et une pression bien prГ©cises, dГ©nommГ©es В« la tempГ©rature et la pression du point triple В».
triple point Chem the temperature and pressure at which the three phases of a substance are in equilibrium. The triple point of water, 273.16 K at a pressure of 611.2 Pa, is the basis of the definition of the kelvin Triple Point in thermodynamics, the point on a phase diagram at which three different phases of a substance can coexist in equilibrium. It Fig. 5.1: Triple point of water Pressure [Pa] Temperature [K] Water Vapor Ice 611.73 273.16 Triple point Melting curve Saturated vapor curve Sublimation
-- The three phases are solid, liquid and gas. There are numerous combinations of pressure and temperature at which a substance can coexist as (solid – liquid), or (liquid – gas) or (gas – solid) phases. But there is only one combination of pres... The triple point of water or any substance is the combination of temperature and pressure at which it exists in its solid, liquid, and gaseous state all at once, in equilibrium. The temperature of the triple point of water is a commonly used physical constant which is used to define temperature scales and calibrate temperature-measuring systems.
Fig. 5.1: Triple point of water Pressure [Pa] Temperature [K] Water Vapor Ice 611.73 273.16 Triple point Melting curve Saturated vapor curve Sublimation -- The three phases are solid, liquid and gas. There are numerous combinations of pressure and temperature at which a substance can coexist as (solid – liquid), or (liquid – gas) or (gas – solid) phases. But there is only one combination of pres...